Top 20 Most Important Alcohol, Phenol & Ether Questions for Class 12 | CBSE & NEET 2025
✨ Are you preparing for CBSE Class 12 Chemistry or NEET? This blog contains the top 20 questions from Alcohols, Phenols & Ethers chapter with detailed handwritten solutions. ✅ Based on NCERT + previous year trends. A must-practice set for scoring high in boards & entrances! 📘
Q1. write the IUPAC name of the following:
Ans. 3,3-dimethyl-pent-2-ol
Q3. Write the IUPAC name of the following:-
Ans. 3-phenylprop-2-en-1-ol
Q4. Write the IUPAC name of the following:-
Ans. 2-phenylethanol
Q5. Write the IUPAC name of the following:-
Q6. Write the IUPAC name of the following:-
Ans. 2,5-dintrophenol
Q7. Write the IUPAC name of the following:-
Q8. Write the IUPAC name of the following:-
Ans. 3-methylbut-2enol
Q9. Write the IUPAC name of the following:-
Ans. Butan-2-ol
Q10. Write the IUPAC name of the following:-
Common name(glycerol)
Q11. Carry out the following conversion: phenol to salicylaldehyde
Ans.
Q12. Carry out the following conversion: propene to propanol
Ans.
Q13. Predict the reagent for carry out the following conversion:phenol to benzoquinone
Ans.
Q14. Predict the following conversion: phenol to 2,4,6-tribromophenol
Ans.
Q15. Predict the following conversion: cumene to phenol
Ans.
Q16. Predict the following conversion: phenol to chlorobenzene
Ans.
Q17. Predict the following conversion: sodium phenoxide to 2-hydroxybenzoic acid
Ans.
Q18. Write the Kolbe's reaction.
Ans.
Q19. Predict the following conversion: Ethanol to Ethanenitrile
Ans.
Q20.Why phenol is more acidic than alcohol?
Ans. Phenol is more acidic than phenol is because when we remove hydrogen ion from phenol then the phenoxide ion shows resonance so it is stable where as in alcohols if we remove hydrogen ion there is no resonance stability hence phenol is more acidic than alcohol.









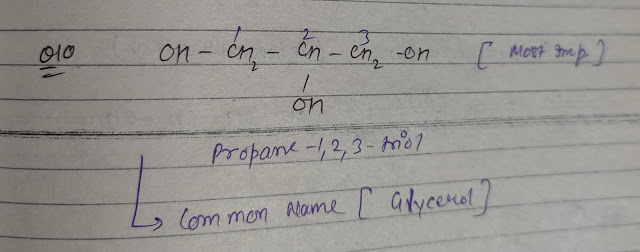








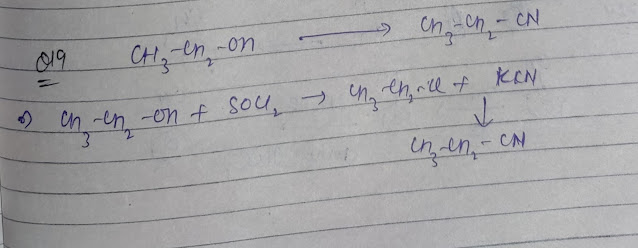






-min.png)





