Top 10 NCERT Questions on Aldehydes, Ketones & Carboxylic Acids
Looking for top NCERT questions from Aldehydes, Ketones & Carboxylic Acids for JEE/NEET? Here are the 10 most important QnA, perfectly suited for quick revision.
Q1. Write the iupac name of the following ketones and aldehyde:
(i)
Ans.Heptan-2-al
(ii)
Ans. 4-Bromo-2-methylhexanal
(iii)
Ans. 3-phenylprop-2-enal
(iv)
Ans. Cyclopentane-1-carbaldehyde
(v)
Ans. Pent-2-enal(vi)
Ans. BenzophenoneQ2. Draw the structures of the following:
(i) 4-methlypent-3-en-2-one
Ans.
(ii) p-methylbenzaldehyde
Ans.
Q3. Arrange the following compound in the increasing order of boiling point.
CH3CHO,CH3CH2OH,CH3OCH3,CH3CH2CH3.
Ans. CH3CH2CH3<CH3OCH3<CH3CHO<CH3CH2OH
Q4. Arrange the following compound in the increasing order of their reactivity nucleophilic addition reaction .
(i) ethanol,propanol,propanone,butanone
Ans. Butanone<propanone<propanal<ethanal
(ii) Benzaldehyde,p-tolualdehyde,p-nitrobenzaldehyde,acetophenone
Ans. Acetophenone<p-tolualdehyde<Benzaldehyde<p-nitrobenzaldehyde
Q5. Complete the following reactions:
(i)
Ans.(i) ClCH2COOH,FCH2COOH,CH3COOH
Ans. CH3COOH<ClCH2COOH<FCH2COOH
(ii) CH3CH2CH(Br)COOH,CH3CH(Br)CH2COOH,(CH3)2CHCOOH,CH3CH2CH2COOH
(iii)Benzoic acid, 4-nitrobenzoic acid, 3,4-dinitrobenzoic acid, 4-methoxy benzoic acid
Q7. Although phenoxide ion has more number of resonating structure than carboxylate ion. Carboxylic acid is a strong acid than phenol, Give reason.
Phenoxide ion has more number of
resonating structure than carboxylate ion but carboxylic acid is more acidic than phenol it is because in the resonating structure of phenoxide ion carbon atom has negative charge which is highly unstable while in the resonating structure of carboxylate ion there is negative charge on oxygen atom which is highly stable as compared to carbon so that is why carboxylic acid is more stable than phenol.
Q8. Give simple chemical test to distinguish:
(i) Acetophenone and benzophenone
Ans. When we react acetophenone with sodium hypoiodite it gives a yellow precipitate where as benzophenone do not.
(ii) Phenol and benzoic acid
Ans. When we react sodium bicarbonate with benzoic acid it will give a brisk effervescence while in phenol no reaction occur .
Alternate method
(iii) benzoic acid and ethly benzoate
Ans. When we react sodium bicarbonate with benzoic acid it will give a brisk effervescence while in ethyl benzoate no reaction occur .
(iv) Pentan-2-one and Pentan-3-one
Ans. When we react pentan-2-one with sodium hypoiodite it gives a yellow precipitate where as Pentan-3-one do not.
(v) ethanal and propanal
Ans. When we react ethanal with sodium hypoiodite it gives a yellow precipitate where as propanal do not.
Q9. Describe the following:
(i) Acetylation:-
Ans. Acetylation is the process of introducing an RCO-- group in compound that contain a replaceable hydrogen atom.
(ii) Cannizaro reaction:-
Aldehyde ketone which doesn't have an alpha hydrogen . Such carbonyl compounds in the presence of conc. NaOH and heat undergo disproportionation reaction to produce corresponding carboxylate ion and alcohol.
Q10. Complete the following reactions:
🟢Conclusion :
Practice these NCERT QnA to strengthen your understanding and score better in JEE/NEET. Bookmark and share with fellow aspirants! 🚀

































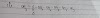
-min.png)





